

Usually, a chemical that is active at the interface between the two phases is used. The viscosity decrease is usually accompanied by a decrease in the interfacial tension, more readily making a good emulsion form.Ī stable emulsion of two immiscible liquids is rare, and some type of chemical assistance is typically required. The simplest way to achieve a viscosity reduction is to heat the product because most liquids become less viscous when they heated. This is accomplished in two primary ways: By reducing the viscosity of the internal phase and through the use of chemical additives. It is the activity at this interface that occupies the majority of this article.Ĭlearly, since the interfacial tension is a measure of the forces trying to keep the two phases separate, the goal in preparing emulsions must be to reduce the interfacial tension to promote a more intimate blending of the two phases. These forces result in an interfacial tension between the two phases. The molecules of both phases at this interface also experience forces that tend to bind them to their own kind and make breaking a single droplet into multiple smaller droplets difficult. This surface, which represents a boundary between the two phases, is called an interface. How does this concept apply to emulsions? Consider the surface of the droplets comprising the internal phase.
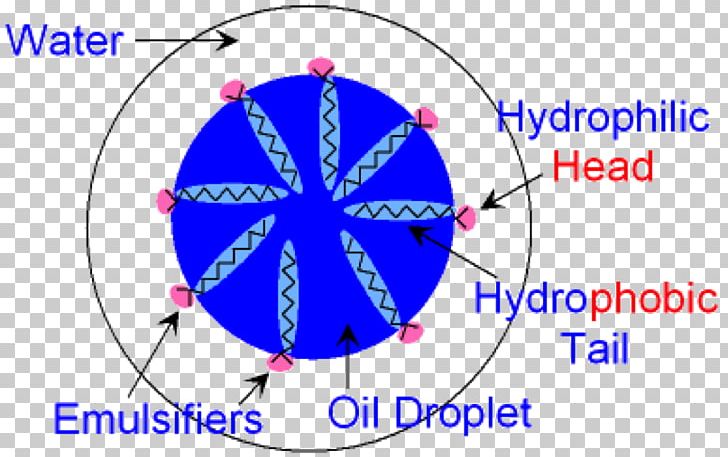
This is the reason that a glass of water can be carefully filled so that the surface bulges above the top of the glass without spilling. The molecules on the surface experience forces that tend to bind them to the bulk of the liquid and prevent their escape into the air. Most people are at least somewhat familiar with the concept of surface tension in which liquid in a container acts as if it has a skin. In the latter, the internal phase is the water-like liquid, while the external phase is the oil-like liquid. In the former, the internal phase is an oil or oil miscible liquid, and the external phase is water or a water miscible liquid. They are called oil-in-water (O/W) and water-in-oil (W/O) emulsions. The liquid present as the surrounding medium is the external phase or continuous phase.īased upon these distinctions, two general types of emulsions are possible. This is accomplished by designating the liquid that exists in the form of small droplets as the dispersed phase or internal phase. Since an emulsion consists of two distinct fluids, distinguishing between them is important.
#Emulsion chemistry full#
While this seems like a simple definition, its full scope will become evident.


Many such definitions are possible, but the most basic one defines an emulsion as a stable mixture of two immiscible liquids, one of which is uniformly dispersed in the other in the form of small droplets or particles. The logical place to begin is with a working definition of an emulsion. This article will discuss basic topics that will assist in the successful development and final evaluation of stable emulsions – including definition of the relevant terms, proper formulation of the product, recommended premix methods, optimization of the processing equipment and methods of evaluating the finished product quality. The use of colloid mills and in-line mixers is a popular way to prepare and process emulsions. A manufacturer that uses a tooling lubricant to produce aircraft engine parts or someone applying a cosmetic cream are common examples of emulsion use. The development and processing of emulsions is common in many industries. Emulsions are found in every aspect of daily lives.


 0 kommentar(er)
0 kommentar(er)
